Scientists Develop Multifunctional Device Which Combines Hemorrhage Treatment and Monitoring Capabilities
(LOS ANGELES) – June 28, 2023 - A multi-faceted device for effectively treating deep, non-compressible, and irregularly-shaped wounds has been engineered by the scientists at the Terasaki Institute for Biomedical Innovation (TIBI). As outlined in their recent paper in Advanced Science, the device provides rapid hemorrhage management, has minimal inflammatory effects, and provides infection control. It also has tunable biodegradation rates, making it usable for both internal and external use, and features sensing capabilities for long-term hemorrhage monitoring. This versatile device is highly beneficial for timely alerts and control of bleeding from surgical wounds, traumatic injuries, and critical illnesses.
There are currently many hemorrhage control products available such as cotton or gauze bandages, powders, and tourniquets. These are usually used with compression on shallow, more uniformly shaped wounds, and they cannot simultaneously detect bleeding and control hemorrhage. Newer, highly absorbent shape-memory sponges treat deep, irregularly shaped wounds while retaining their structure and original dimensions; after blood absorption, these sponges also naturally apply pressure to the wound and promote coagulation. Cellulose-based shape-memory sponges are adequate but not biodegradable as gelatin-based versions are, and neither have the ability for hemorrhage detection.
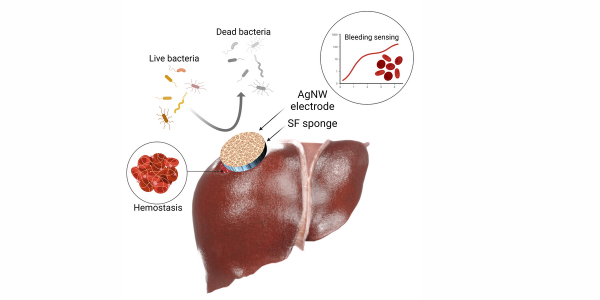
To create a versatile hemorrhage management device, the TIBI team turned to silk fibroin, a protein produced by the Bombyx mori silkworm. Silk fibroin is a biodegradable material with optimum anti-inflammatory and mechanical properties and can be engineered into porous, highly absorbent memory-shaped sponges. These sponges can also promote coagulation and tissue regeneration. The tunable degradation rates of the sponges allow longer-term use in the body as well as the possibility of integration with sensors that can monitor bleeding over time.
The TIBI team leveraged these capabilities and developed a unique, all-in-one hemorrhage management device. The device consists of two silver nanowire layers positioned above and below a hemostatic sponge layer. The nanowires function as hemorrhage detection sensors as well as antibacterial agents.
The TIBI team carried out a comprehensive evaluation of the device, including testing mechanical properties, biocompatibility, and biodegradation. Mechanically, the SF sponges showed outstanding elasticity and absorptive powers, along with excellent retention of pore size, shape, and size when tested against water and blood; optimum hemorrhage control could be obtained by tuning the silk fibroin concentrations to match the mechanical properties of surrounding wound tissue. Favorable biocompatibility and minimal anti-inflammatory responses in both the sponge and nanowire layers were shown by good cellular viability and proliferation when tested with connective tissue samples. Further tests showed that the degradation rate of the sponge could be slowed both by increasing the silk fibroin concentration and by employing a methanol wash step during fabrication.
Next, the device and a commercial gelatin-based anti-hemorrhage device were evaluated in rat models via below-the-skin implantation. The commercial sponge completely degraded after four weeks, while the silk fibroin nanowire device maintained its structure. In addition, the implanted sponge showed minimal inflammatory responses and posed no adverse effects on the organs and the behavior of the rats. Also, the silk fibroin device outperformed the commercial sponge in hemorrhage control tests, with a two-fold higher level of hemorrhage control in a rat bleeding model.
In addition to providing hemorrhage management, the device also includes a nanowire-based capacitive sensor for bleeding detection. During bleeding, the sponge absorbs the blood, which increases its capacitance without affecting its shape. The increase in capacitance is detectable and directly correlates to the amount of blood absorbed, thus providing a way to monitor bleeding in real time. Tests showed that the device could selectively monitor blood absorption against other bodily fluids it might encounter in the wound.
“This multifunctional device offers many attractive features for hemorrhage control and wound monitoring and is highly adaptable for different types of wounds and tissues,” said Ali Khademhossein, Ph.D., TIBI’s Director and CEO. “And the hemorrhage monitoring feature also opens up several possibilities for integrative biosensing and additional therapeutics.”
Authors are: Reihaneh Haghniaz, Ankit Gangrade, Hossein Montazerian, Fahimeh Zarei, Menekse Ermis, Zijie Li, Yuxuan Du, Safoora Khosravi, Natan Roberto de Barros, Kalpana Mandal, Ahmad Rashad, Fatemeh Zehtabi, Jinghang Li, Mehmet R. Dokmeci, Han-Jun Kim, Ali Khademhosseini, Yangzhi Zhu.
This work was partially supported by the National Institutes of Health (AR074234, AR073135, HL140618, HL137193, GM126571, GM126831).
DOI: https://doi.org/10.1002/advs.202301406